
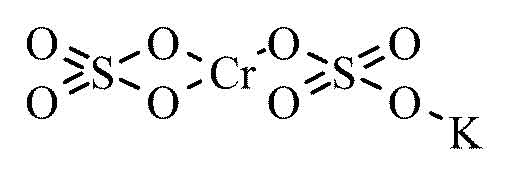
The latter control solution is contained in another compartment in the Breathalyzer ®.
To determine the degree of the color change, and so calculate the alcohol level, it is necessary to compare the test solution to an unreacted solution. The degree of the color change corresponds to the amount of alcohol that is present. In this reaction, the alcohol (ethanol) reacts with the reddish-orange potassium dichromate to produce the greenish-colored chromium sulfate, potassium sulfate, and acetic acid. In the solution, the silver nitrate acts as a catalyst (a compound that speeds up the rate of reaction without directly participating in the reaction) in the reaction that follows. The sulfuric acid acts to remove the alcohol from the air into the aqueous solution. When air is blown into a Breathalyzer ®, it bubbles through a mixture of potassium dichromate, sulfuric acid, silver nitrate, and water. This calculation is done as described below. By determining the amount of alcohol in the volume of expired air, a calculation of blood alcohol concentration can be made. The ratio of the amount of alcohol in the breath to the blood alcohol level is 2,100:1. When someone blows into a Breathalyzer ®, the device detects both the volume of air expired and, as described below, the amount of alcohol present. There, alcohol ’s volatility encourages its passage across the channel membranes into the lung, where it can be exhaled.Ī Breathalyzer ® detects this expired alcohol. The latter is important when alcohol-laden blood passes through the tiny channels in the air sacs of the lungs. The Breathalyzer ® relies on the fact that, when consumed, alcohol is not altered in the bloodstream and on the volatility of the compound (the tendency of the compound to evaporate from solution). Borkenstein of the Indiana State Police invented the modern-day Breathalyzer ®. The development of the devices that could monitor alcohol in the breath dates back to the 1940s. If they were driving a vehicle, they would be charged with driving while impaired (DWI) or driving under the influence (DUI). So, for example, if a suspect ’s breathalyzer reading was 0.15%, they would be legally impaired. The legal BAC limit can vary between jurisdictions 0.08% is a typical limit. The result is typically expressed as the blood-alcohol concentration (BAC), which represents the grams of alcohol per 100 milliters of blood. The instrument uses a color reaction to detect alcohol the degree of color change is related to the alcohol level in the breath. It is the most popular device for portable, at-the-scene use. The Breathalyzer ® is one of three different devices that can be used. Measurements of alcohol level are most conveniently taken by monitoring expired air. But, determination of blood alcohol levels via a Breathalyzer ® test is a critical part of the assessment of sobriety.Īn initial Breathalyzer ® measurement is often conducted at the scene of the accident, since normal metabolic processes in the body will reduce the alcohol level within hours. Several tests of coordination (i.e., walking a straight line, touching the tip of the nose with a finger) can be helpful indicators. Often a police officer needs to ascertain whether a person is legally impaired. Returning to the example of a traffic accident, one of the early facets of an investigation can be the determination of the alcohol level of a driver. The determination of alcohol level can be an important part of an ongoing investigation. In fact, alcohol-related traffic accidents are the leading cause of death among Americans aged 16 –24. In the United States, at least 25,000 people die in alcohol related traffic accidents each year, representing about 500 people each week. Accidents or crimes can occur when one or more of those involved are intoxicated.


 0 kommentar(er)
0 kommentar(er)
